UPNEEQ®
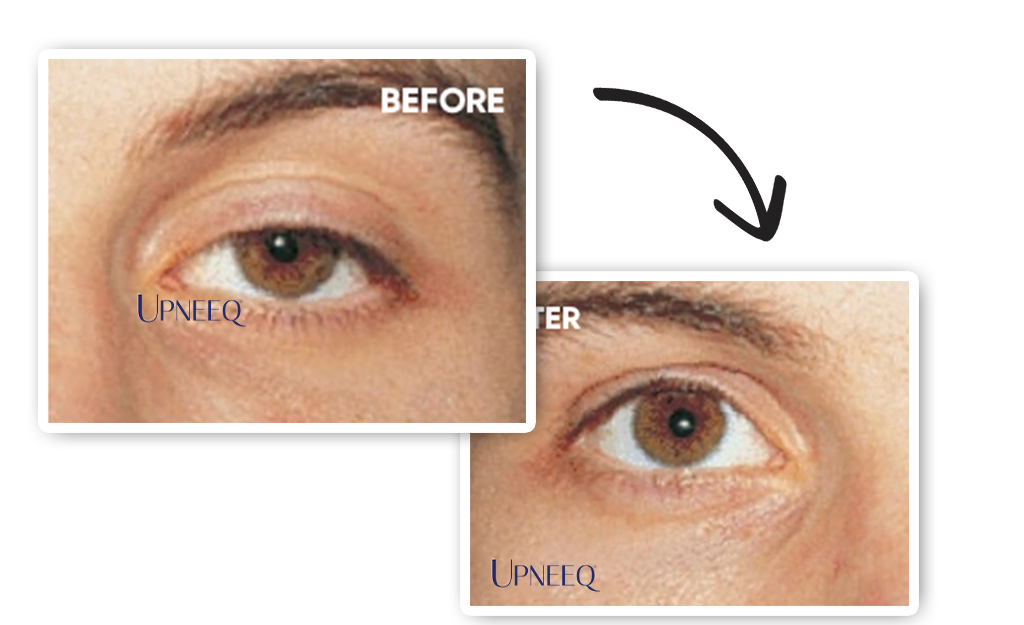
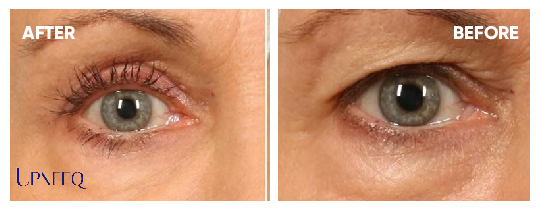
Upneeq® is the only FDA-approved prescription eyedrop for acquired ptosis (low-lying lids) that lifts your upper eyelids to open your eyes.
- Non-invasive eye lift
- Lasts up to 8 hours
- Use at your own pace

5 Min.
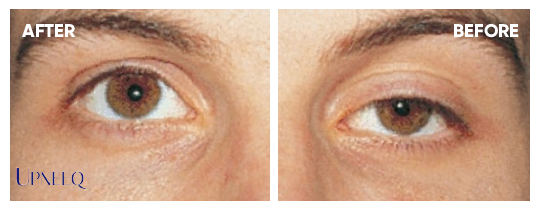
In one study, some patients saw a lift in their eyelids as fast as 5 minutes after the first dose.
84%
of patients had some form of improvement.
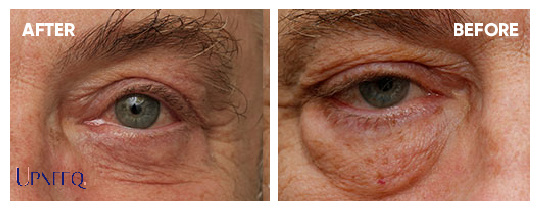
74%
of patients had some more than a 50% improvement.
5 Mins.
In one study, some patients saw a lift in their eyelids as fast as 5 minutes after the first dose.
Medical History Form






Questions? We're here to help.
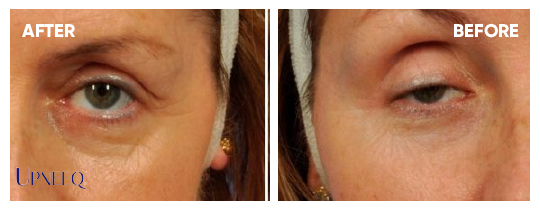
UPNEEQ® (oxymetazoline hydrochloride ophthalmic solution), 0.1% is a prescription eyedrop used to treat low-lying lids (acquired blepharoptosis) in adults.
The most common adverse reactions with UPNEEQ® (occurring in 1-5% of patients) were eye inflammation, eye redness.
In the trial, the onset of the drop was statistically significant at 15 minutes, although many patients responded as early as 5 minutes.
If you wear contact lenses, remove them before instilling Upneeq eye drops. You may put them back in 15 minutes after administering Upneeq.
If more than one medication or eye drop is being used in the eye, they should be administered at least 15 minutes apart.
Step 1: Cut open foil wrapper and remove single-use vial
Step 2: Apply one drop of UPNEEQ® in each affected eye as directed, once a day. Do not let the tip of the vial touch your eye or any other surface. Vials should not be re-usedafter opening and should be thrown away after applying drop(s)
- If you wear contact lenses, remove them before applying UPNEEQ®eyedrops
- You may put them back in 15 minutes after applying UPNEEQ®
- If more than one topical ophthalmic drug is being used, the drugs should be administered at least 15 minutes between applications
Most patients in clinical trials had a lift in their eyelids for 6–8 hours.
HOW IT WORKS

Select Upneeq Size
You always have the option to purchase one time or subscribe to lock in extra savings

Fill Out The Required Medical Form
Complete our secure medical intake form online and one of our medical providers will review
- Takes 5 mins

Upload Photo Or Complete Video Consultation
Provide a current photo of your full face and an image of your valid, state-issued ID or Complete Video Consultation*
*Telemedicine law varies from state to state
Information



